The titration of 80.0 mL of an unknown is a fundamental technique in analytical chemistry that allows us to determine the concentration of an unknown solution. This process involves the careful addition of a known volume of a titrant solution to the unknown solution until the reaction between them is complete.
By monitoring the change in the solution’s properties, such as pH or color, we can determine the equivalence point, which corresponds to the complete reaction of the unknown solution. This guide will provide a comprehensive overview of the titration process, including the equipment used, the step-by-step procedure, and the data analysis involved.
The determination of unknown concentrations is crucial in various fields, including chemistry, environmental science, and medicine. Titration plays a vital role in quality control, drug analysis, and environmental monitoring, making it an essential technique for researchers and practitioners alike.
Overview of Titration
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The process involves gradually adding the known solution (titrant) to the unknown solution (analyte) while monitoring the reaction using an indicator or an instrument.
The basic principle of titration is to reach an equivalence point, where the moles of the titrant added are equal to the moles of the analyte present in the solution. This point is often indicated by a color change in the indicator or a change in the instrument’s reading.
Titration experiments typically use equipment such as a burette, pipette, flask, and indicator.
Unknown Concentration Determination
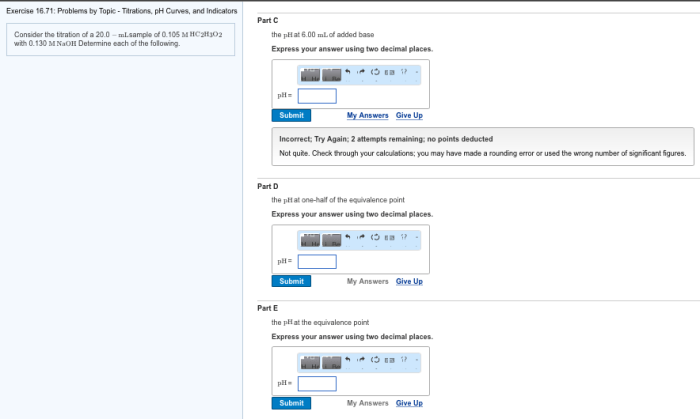
Determining the concentration of an unknown solution is important in various fields of science, including chemistry, environmental science, and medicine.
Titration plays a crucial role in determining the concentration of an unknown solution by providing a precise and accurate method to measure the amount of analyte present in the solution.
Titration Procedure
Preparation of the Unknown Solution, The titration of 80.0 ml of an unknown
The unknown solution is prepared by dissolving a known mass of the analyte in a known volume of solvent.
Preparation of the Titrant Solution
The titrant solution is prepared by dissolving a known mass of the titrant in a known volume of solvent. The concentration of the titrant solution is accurately known.
Setup of the Titration Apparatus
The titration apparatus consists of a burette, pipette, flask, and indicator. The burette is used to deliver the titrant solution, the pipette is used to measure the volume of the unknown solution, the flask is used to hold the unknown solution, and the indicator is used to signal the equivalence point.
Monitoring and Recording of Titration Data
The titration is performed by gradually adding the titrant solution to the unknown solution while constantly stirring. The volume of titrant added and the corresponding observations (e.g., color change of the indicator) are recorded.
Data Analysis and Calculations
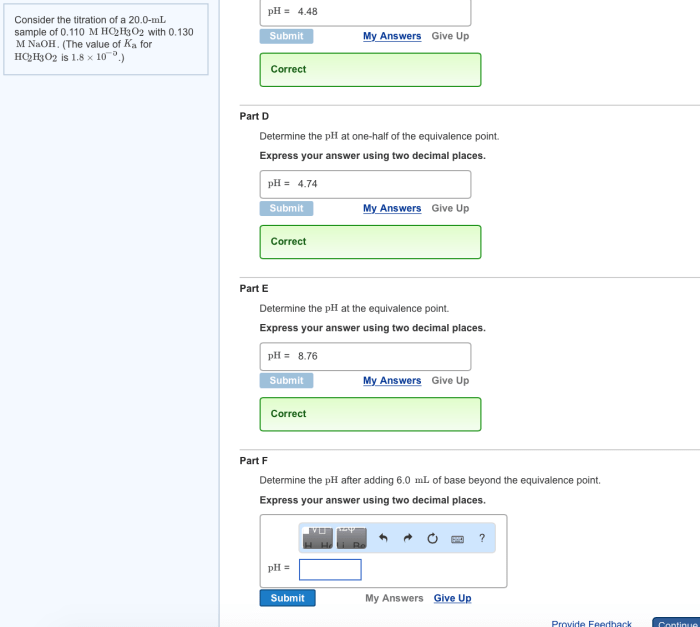
The titration data is analyzed to determine the unknown concentration using the following formula:
M1V1 = M2V2
where:
- M1 is the molarity of the titrant solution
- V1 is the volume of the titrant solution used
- M2 is the molarity of the unknown solution
- V2 is the volume of the unknown solution
Precision and accuracy are crucial in titration experiments. Precision refers to the reproducibility of the results, while accuracy refers to the closeness of the results to the true value.
Common Titration Techniques
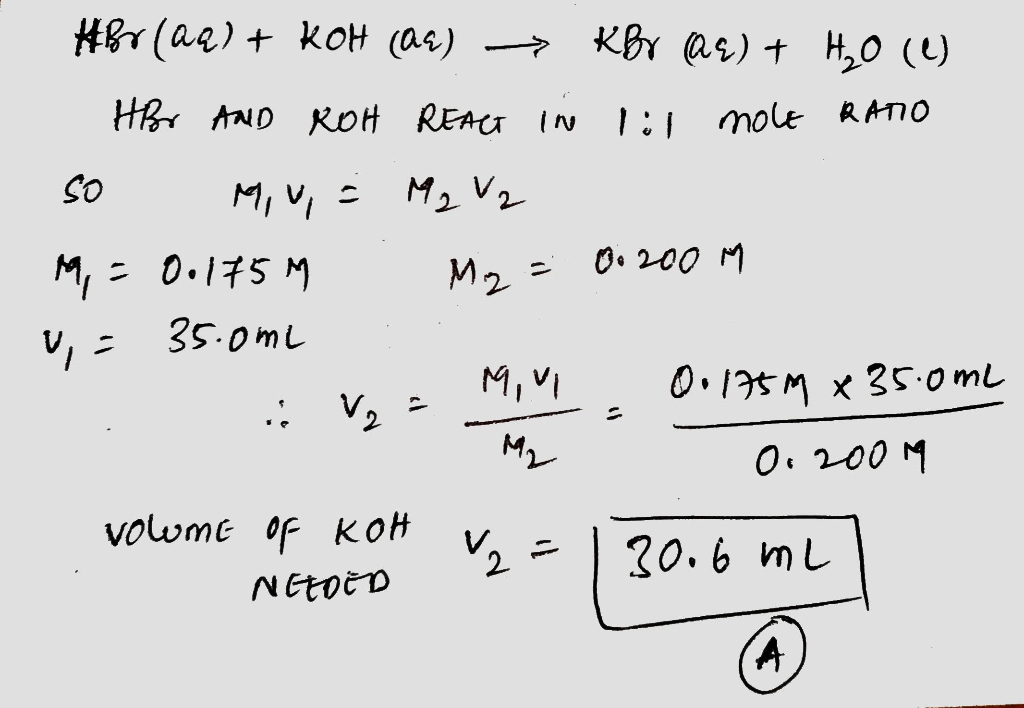
Acid-Base Titration
Acid-base titration involves the reaction between an acid and a base to determine the concentration of either the acid or the base.
Redox Titration
Redox titration involves the reaction between an oxidizing agent and a reducing agent to determine the concentration of either the oxidizing agent or the reducing agent.
Precipitation Titration
Precipitation titration involves the reaction between two ions in solution to form a precipitate. The concentration of one of the ions can be determined by measuring the amount of precipitate formed.
Applications of Titration
Chemistry
Titration is widely used in chemistry for various purposes, such as:
- Determining the concentration of acids and bases
- Analyzing the purity of chemicals
- Studying reaction kinetics
Environmental Science
Titration is used in environmental science to:
- Measure the concentration of pollutants in water and soil
- Monitor the effectiveness of water treatment processes
- Assess the impact of industrial activities on the environment
Medicine
Titration is used in medicine to:
- Determine the concentration of drugs in blood and urine
- Analyze the acidity or alkalinity of body fluids
- Monitor the effectiveness of medical treatments
Q&A: The Titration Of 80.0 Ml Of An Unknown
What is the purpose of titration?
Titration is used to determine the concentration of an unknown solution by reacting it with a known volume of a titrant solution.
What is the equivalence point in titration?
The equivalence point is the point at which the moles of titrant added are equal to the moles of analyte in the unknown solution.
What are the different types of titration techniques?
There are various titration techniques, including acid-base titration, redox titration, and precipitation titration.
What are the applications of titration?
Titration is widely used in chemistry, environmental science, and medicine for quality control, drug analysis, and environmental monitoring.