Naming binary compounds covalent worksheet – Introducing the “Naming Binary Covalent Compounds” worksheet, an invaluable resource designed to enhance your understanding of covalent bonding and nomenclature. This engaging guide provides a comprehensive overview of the topic, empowering you to master the intricacies of naming binary covalent compounds with confidence.
Delve into the fundamental concepts of covalent bonding, exploring the properties and characteristics of these fascinating compounds. Discover the IUPAC guidelines for naming binary covalent compounds, unlocking the secrets behind their systematic nomenclature.
1. Understanding Covalent Compounds
Covalent compounds are chemical compounds formed when two or more non-metallic elements share electrons to achieve a stable electron configuration.
Properties of covalent compounds include:
- Low melting and boiling points due to weak intermolecular forces
- Poor conductors of heat and electricity
- Tend to be gases or liquids at room temperature
- Examples include hydrogen (H2), water (H2O), and methane (CH4)
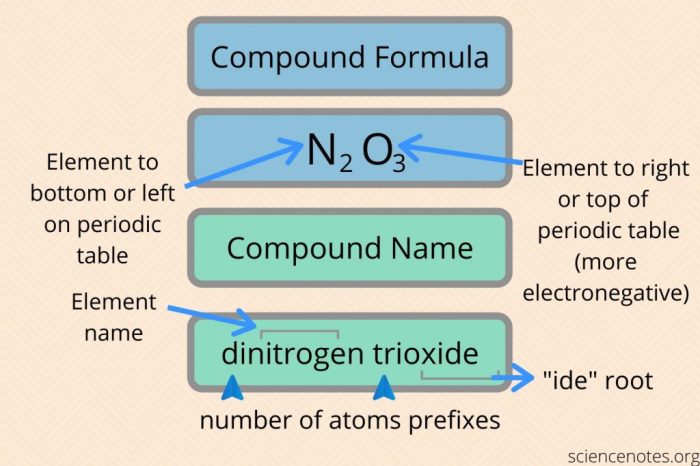
2. Nomenclature of Binary Covalent Compounds
Binary covalent compounds consist of two non-metallic elements. According to IUPAC guidelines, they are named using the following steps:
- Identify the more electronegative element and name it as the second part of the compound, adding the suffix “-ide”.
- Identify the less electronegative element and name it as the first part of the compound, using prefixes to indicate the number of atoms present (mono-, di-, tri-, etc.).
- Use hyphens to separate the two elements.
For example, the compound composed of one carbon atom and four hydrogen atoms is named “methane”.
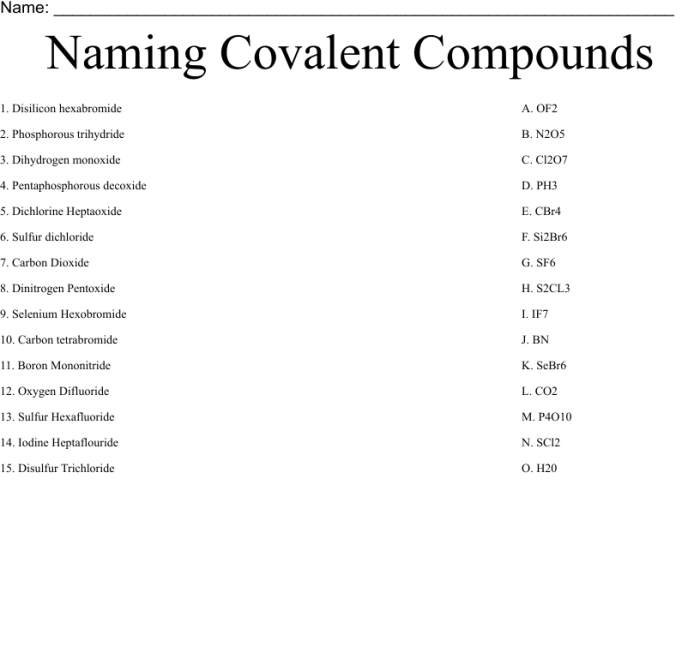
3. Worksheet Activities
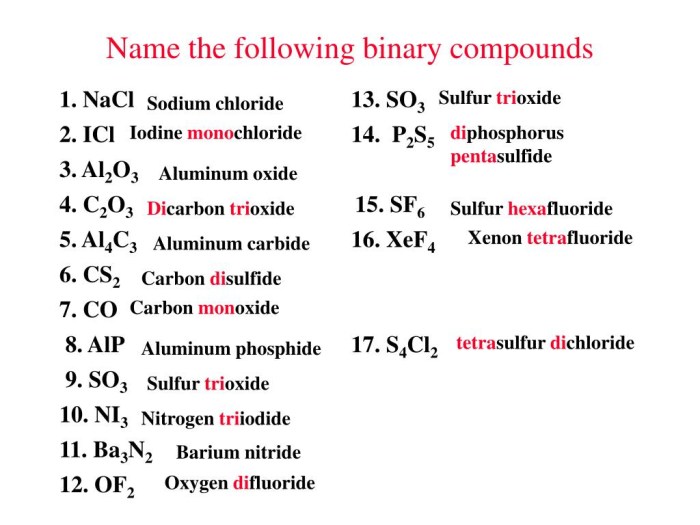
The worksheet includes practice problems for naming binary covalent compounds. It is designed to cater to different learner levels and provides answer keys for self-assessment.
4. Applications of Binary Covalent Compounds
Binary covalent compounds have numerous applications in various fields:
- Chemistry:As reactants and products in chemical reactions
- Electronics:As semiconductors in transistors and integrated circuits
- Medicine:As drugs, anesthetics, and diagnostic agents
Examples include silicon dioxide (SiO2) used in glass and ceramics, and carbon dioxide (CO2) used as a fire extinguisher and refrigerant.
5. Additional Resources: Naming Binary Compounds Covalent Worksheet
- Covalent Bonds (Khan Academy)
- Covalent Bond (Encyclopedia Britannica)
- Nomenclature of Inorganic Chemistry (IUPAC Red Book)
FAQ Guide
What are the key features of covalent bonding?
Covalent bonding involves the sharing of electron pairs between atoms, resulting in the formation of stable molecules.
How does the IUPAC system guide the naming of binary covalent compounds?
The IUPAC system assigns prefixes to indicate the number of atoms of each element, followed by the root name of the nonmetal and the suffix “-ide”.
What are some common applications of binary covalent compounds?
Binary covalent compounds find widespread use in industries such as electronics, medicine, and materials science, forming the basis of semiconductors, pharmaceuticals, and plastics.